符号逻辑,也称为形式逻辑或数理逻辑,是逻辑学的一个…
错误检测和纠正码是用于在数字通信中检测和修正数据传…
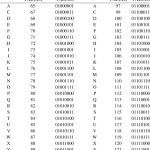
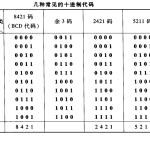
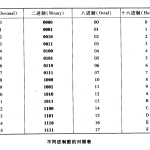
二进制编码是指在二进制系统中表示的代码,与原始代码…
二进制转十进制 任何二进制数都可以通过将二进制数中…
数字数制是指用数字来表示数值的体系,其中最常见的包…
数字电子技术是一种电子技术领域,涉及使用数字信号和…
到目前为止,您应该已经安装了所有软件并构建了界面,…
为了让微控制器做一些有用的事情,我们必须为其编写指…
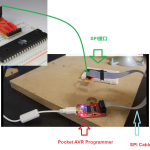
到目前为止,您应该已经构建了SPI接口。如果还没有…
因此,到目前为止,您应该对微控制器(MCU)的概念…
单片机(Microcontroller)是一种集成…
古人云:“湛湛青天不可欺,未曾起意神先知”。贪好色…
每当美联储为应对高通胀而提高利率时,经济衰退的风险…
有轮回转世的研究案例显示,一名比利时女童拥有遥视(…
有哪些从外国传入,却误以为是中国发明的? 有很多东…
长期以来,科学家们都对一种称之为石墨炔的新型碳感兴…
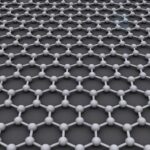
石墨烯(Graphene)是一种由碳原子以sp2杂…
化学气相沉积法(CVD)是一种在相对而言比较高的温…
生成式人工智能靠台积和英伟达不够!日本半导体推手甘…
通常在新的一年到来之前,每个人也都会有新的计划。不…
比特币专家、知名财经记者Max Keiser近日就…
目前全球唯一能够制造EUV光刻机的厂商,只有荷兰的…
FPGA 是现场可编程门阵列的简称。它是一种半导体…
单纯从电脑知识角度来讲,IC是英语integrat…
DSP是(digital signal proce…